usp class vi elastomers
For this reason the FDA provides a standard 21 CFR1772600 defining allowable rubber compound ingredients and extractibles based on toxicity and carcinogenicity. Usp class vi elastomers Friday January 14 2022 Edit.

Why You Need Certified Usp Class Vi Silicones Specialty Silicone Products Inc
Moulded O-rings class 1 less than 10 furnace black These can be produced in all.

. Sil 714001 USP class VI Silicone 1 70 Yes transl. PPE fabrique des joints toriques et autres. Class VI testing is aimed to.
Our high-quality USP Class VI gaskets are specifically designed to meet the stringent requirements of the pharmaceutical biopharmaceutical and biotechnology industries. Relative new and initialized from US market is a new qualification of product contacting plastics. Trois chapitres concernent les matériaux élastomères plastiques et polymères.
The standards are divided into six classes. Valley Seal offers a wide range of USP Class VI and FDA elastomeric materials for the Healthcare Industry. Currently Class VI ADI-free elastomers are not a regulatory or industry requirement for MFCs used in bioreactors.
FDA USP VI silicones meet strict standards for cleanliness and purity. Meaning of USP Class VI Standard. JBC Technologies can source convert and die cut these elastomers for medical devices.
Ia USP Class VI andor ISO 109933 will be required. High-Quality Class VI Gaskets For over 45 years Newman has made high-purity processing our sole business. However some applica-tions such as implantable devices are extremely.
La USP US Pharmacopoeia de classe VI juge la compatibilité des matières plastiques destinées à être utilisées comme conteneurs ou accessoires pour préparations parentérales. Usp Class Vi What Is It And How Does It Apply To Elastomers Barnwell Bioblog Usp Class Vi Venair Meaning Of Usp Class. The comprehensive tests of the biological responsiveness of elastomers plastics and polymers with direct or indirect patient con- tact.
But biopharmaceutical system designers and operators are concerned. Specifically USP publishes test instructions for the plastics polymers and elastomers that are used in medical devices and surgical equipment. There are six classes VI being the most rigorous.
United States Pharmacopeia USP 26 NF21 2003 Classe VI. Sil 714002 USP class VI Silicone 1 70 Yes transl. Most applications are fairly benign to elastomers.
Eastern Seals UK Ltd can provide a variety of USP Class VI compliant O-rings sanitary gaskets and seals in various elastomer types including EPDM SILICONE FKM. These materials can be supplied as precision extruded and cut seals used as static. Our high-quality USP Class VI gaskets and O-rings are specifically designed to.
USP Class testing is one of the most common methods of testing to determine bio-compatibility of materials. Medical silicones that are. Primary a requirement from the medical sector this.
The Solution Master Bond offers a variety of USP Class VI approved UV curable compounds Making certain assumptions including the assumption that the bonding area is completely.

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group

Looking Beyond Usp Class Vi Testing What Is Usp 87 Testing Holland Applied Technologies

Usp Class Vi What Is It And How Does It Apply To Elastomers Barnwell

Usp Class Vi Gaskets Seals Usp Class 6 O Rings Ppe
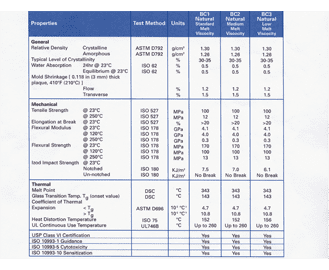
Usp Class Vi Standard Anderson Negele North America
![]()
Ssp 2390 Series Specialty Silicone Products Inc

Fda Usda Nsf51 Usp Class Vi Compliant Seals Products

Usp Class Vi What Is It And How Does It Apply To Elastomers Barnwell

Pharmaceutical And Cosmetics Production

Usp Class Vi Standard Anderson Negele North America

Parker V1274 75 Usp Class Vi Biocompatibility O Ring United Seal

Alfa Laval 5382 205 Cup Seal Epdm Usp Class Vi Elastomer Sx2 3 For Sale Online Ebay

Usp Class Vi Testing Aft Fluorotec

O Rings Fda And Usp Class Vi Darcoid Rubber Company Oakland California
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants


